5 min read
Takeda reduces human performance risk to increase yields
By: Jackie Holen on November 23, 2021

We spoke with Clifford (Cliff) Berry, Head of Business Excellence at Takeda’s Massachusetts Biologics Operations site about Human Performance and how DevonWay software helps them increase pharmaceutical manufacturing yields.
The percent of manufactured batches that are right the first time, aka Batch Right The First Time, is an important measure of quality in pharmaceutical manufacturing: The higher it is, the lower the waste and loss in the manufacturing process.
Japanese multinational pharmaceutical company Takeda implemented DevonWay Observations, internally called Operational Learning Gemba, and DevonWay General Actions, at the Massachusetts Biologics Operations site as part of their Human & Organizational Performance road map. Along with other efforts at the site, the DevonWay solution contributed to a 20% improvement in Batch Right The First Time.
Cliff explained to us what Human Performance means and how their Operational Learning Gemba system works.
What is Human Performance – and Why Is It Important?
“In biotech, Human Performance basically means protecting people and products from harm,” Cliff told us. In pharmaceutical manufacturing, product harm includes issues like product contamination, quality departures, losing data, and even sending the contents of a vessel to a place that it is not intended to go – down the drain or on the floor.
One definition for Human Performance, also called Human & Organizational Performance, is understanding how people interact with plant, processes, and each other – as part of a system, to manage risk to protect people and product from harm by establishing necessary capacities and controls.
“Human Performance as a name can be misleading,” laughed Cliff, “because we are improving systems, not fixing people." In biotech manufacturing, Human Performance is about managing operational risk and failure. It combines domains that include systems safety, human factors, safety science, and resilience engineering.
Cliff has been a Human and Organizational Performance practitioner since 1999 and has experience in biopharma, commercial nuclear electrical generation, electric transmission and distribution, and the US Navy.
Software for Human Performance
When Cliff joined Takeda Massachusetts, the team wrote observations on paper cards, which was time consuming and didn’t provide much-needed visibility. To encourage participation, gain visibility, and improve quality, he knew they needed easy-to-use software to automate observations and follow-up actions.

Cliff reviewed the site’s existing audits and assessments software and realized that it would not work very well for Human Performance. The legacy system was rigid and nonintuitive. Its workflow capabilities weren’t flexible. More importantly, it used terminology that discouraged a Human Performance mindset. Human Performance recognizes that the worker is the solution to the problem, not the problem, so the approach and language are different from traditional inspections, audits, assessments, and corrective and preventive actions programs.
The team needed flexible software that:
- Enables quick changes to fields, pick lists, and workflows to fit their Human Performance program goals and can reflect colleagues’ requests for changes.
- Engages the workforce with easy-to-use interfaces on desktop and mobile.
- Has flexible, configurable workflow options, enabling colleagues to choose who (individuals or teams) needs to do what when.
- Includes business intelligence, trending, and reporting functionality that business users can use without involving IT.
- Provides robust action tracking and configurable notifications to ensure follow-up and support learning.
The DevonWay Human Performance Solution
Cliff turned to DevonWay, a vendor he’d worked with successfully at a prior company. “I know of other vendors that once you’ve paid them, you’re stuck on an island, and they don’t help you. Not with DevonWay – when we need changes, we get fast turnaround.” He often makes changes himself in response to his users’ feedback.
Internally, Takeda calls its observation solution Operational Learning Gemba, because gemba is a common term in manufacturing operations where Lean Six Sigma is used. (“Gemba” is Japanese for “the real place,” now adapted in management terminology to mean the 'workplace' or the place where value is added.)
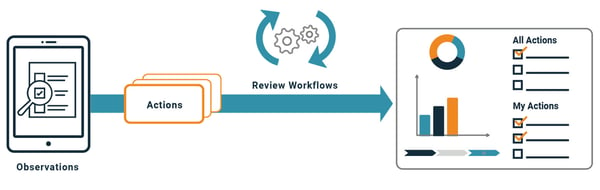
Leaders enter Observations and optionally add follow-up Actions. Actions are assigned, reviewed, and tracked to completion. Users are automatically notified when Actions assigned to them are coming due or overdue. The system distributes weekly management reports by email to the site leadership team and manufacturing managers, who can view the summary information within the email or click to open the record in a browser for more details.
Results: Increased Learning, Improved Outcomes, and Cost Savings
With the DevonWay system, the site leadership team now has visibility into engagement and progress. Cliff tracks time and resources invested in operational learning.
Along with other efforts at the site, the DevonWay solution helped improve Batch Right The First Time by 20%. As deviations that contribute to Batch Right The First Time are addressed through systems improvements, the likelihood of no batches being lost is improved. In pharmaceutical manufacturing, a batch loss can cost $2 million or more, so improvements quickly add up to many millions of dollars saved.
“Two key elements of a Human Performance program are learning and fixing,” Cliff says. “You have to watch work and engage with the experts who perform the work, and Operational Learning Gemba helps our leaders to learn about work in a very effective manner.”
Learn More About Human Performance
Amy Wilson, Ph.D, global human performance lead for Biogen, explains in Pharmaceutical Online that “It’s important to equate human performance with overall performance.... If [you make] it easier to ‘get it right’ and harder to ‘get it wrong,’ you will see overall improvement in success rates and operational metric performance.”
In a video series called Understanding Human Error, Human Performance expert Sidney Dekker says, “Human error is a symptom of trouble deeper inside the system. Human error [should be] a starting point for understanding how people create safety through practice.” It’s not the conclusion.
For guidance on the discipline of Human Performance in pharmaceutical operations, see Human Performance in Biopharma Operations – Your Problem Isn’t Error (November 9, 2020) and Moving Beyond Human Error In Biopharma Investigations and CAPA Programs (November 16, 2020) in Pharmaceutical Online.
DevonWay Software for Pharmaceutical Manufacturing
Visit www.devonway.com/biotechnology for more information about DevonWay software for biotechnology and pharma manufacturing.
Related Posts
Top Compliance Management Challenges & How to Overcome Them
Compliance management can be complex and overwhelming, especially for organizations in high-risk,...
Department of Energy's Waste Isolation Pilot Plant Goes Live with DevonWay Contractor Assurance System Software
DevonWay, a leading provider of asset, quality, and safety management software to highly-regulated...
Independent Research Firm Publishes Case Study on DevonWay EHS, QMS, and Asset Management Solutions
Verdantix, an independent UK-based research and consulting firm with expertise in environmental,...